Specifics for the specialist: Devleopment and intrinsic plasticity of excitable cells.
Weakly electric fish generate electric fields for communication and electrolocation. These electric fields are generated by the discharge of a specialized electric organ. These electric organ discharges (EODs) are the summed action potentials of specialized cells within the electric organ, known as electrocytes. Electric fish are specialists in both the modulation and precise regulation of action potential waveform, making them ideal model organisms for exploring the biochemical and biophysical control of excitable membrane physiology. Sex steroids organize sexual dimorphism in the action potentials of individual electrogenic cells, and electric fish have been shown to rapidly modulate the amplitude and duration of their EOD waveform in a circadian rhythm and in response to changes in immediate social conditions. My work focuses on investigating the cellular mechanisms of EOD generation and plasticity.
Of particular interest to me are the rapid changes seen in electric waveforms, changes that appear too quickly to be accounted for by genomic steroid action. One species that specializes in rapid waveform modulations is Brachyhypopomus pinnicaudatus, a fish that generates a biphasic EOD. This species was first discovered and studied by Carl Hopkins, and also has been intensively investigated in Philip Stoddard's laboratory. We have shown that these changes in EOD waveform are mediated by peptide hormones that act via a cAMP/PKA pathway to modulate the discharge of single electrocytes. Even more interesting, the electrocytes of these fish have two spike-generating excitable membranes that are independently regulated. Generation of the biphasic EOD in this species depends on maintaining precise, microsecond-scale timing between the two action potentials, while also maintaining distinct action potential waveforms form each membrane. Modulations of EOD waveform involve microsecond scale changes in the timing of the two action potenials, as well as the differential modulation of the two action potential waveforms.
Based on these findings, I am pursuing two lines of related research. The first is to investigate the molecular and ionic mechanisms underlying the changes in electrocyte action potentials and spike-timing. Toward this end, I have developed a loose-patch recording procedure for recording isolated ion currents from each of the electrocyte's excitable membranes. These recordings will enable us to determine exactly what biophysical changes are responsible for changes in the timing and waveform of the electrocyte action potentials.
The second line of research is directed toward understanding the development of the electrocyte and its mechanisms of plasticity. Cheryl Franchina showed that, In B. pinnicaudatus, the electrocytes are derived from skeletal muscle. During the first few days of life, skeletal myocytes fuse and lose their contractile properties while retaining their electrical excitability. The result is the larval electrocyte which is a cigar-shaped cell with a single excitable membrane at one end. These larval electrocytes produce a monophasic discharge. During the next 100 days or so, the electrocytes slowly take on a disk-like shape, and develop a second region of excitable membrane. The result is that each face of the electrocyte is excitable and the cell begins producing a biphasic discharge, the result of action potentials on its dual excitable membranes.
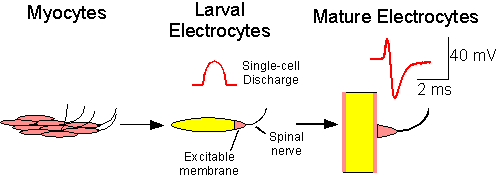 |
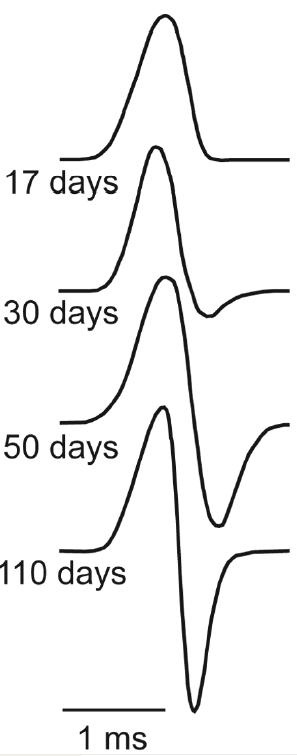 |
This developmental trajectory raises a number of very interesting questions: What ionic currents are gained and/or lost during the transitions from myocyte to larval electrocyte to mature electrocyte? Are modulatory mechanisms present in the myocyte carried forward to enable the plasticity observed in the mature elecrocyte, or are these modulatory mechanisms brought online sometime during development? If the mature electrocytes membranes are populated by different ion channel types as we suspect, how are different ion channels targeted to different membranes in the same cell compartment? The list of questions goes on and on, which illustrates why I believe this is such a fertile system for studying the development and plasticity of excitable membranes.

